
علوم کاربردی
آنسوی مرز های شیمی تئوری
علوم کاربردی
آنسوی مرز های شیمی تئوریپیوندها
دستهها
ابر برجسب
گرمای نهان ذوب دودزا اسید سولفوریک خالص سازی گرمای نهان سیلیکون دی اکسید شیمی کلسیم کربنات آهک سدیم کربنات HNO3 NO2 گوگرد سیلیکون کاربید ترقهمشخصات مواد شیمیایی
- سدیم بی سولفات
- کلسیم کربنات
- اکسید سرب
- کربن دی اکسید و یخ خشک
- سیلیکون کارباید
- سدیم سیلیکات
- کلرات پتاسیم
- پتاسیم کلرید
- سدیم کلرید
- میزان بازده محصول واکنش سدیم و کلر طبق روابط استوکیومتری
- مشخصات آمونیوم نیترات
- مشخصات ترفتالیک اسید
- چار ت درصد ترکیب شدن بوتان و پروپان و نتیجه آن
- چارت برش فلزات با مشعل اکسی استیلن
- دمای شعله گاز های مختلف
- Sodium acetate
- سرکه یا استیک اسید
- جدول پتانسیل استاندارد یا جدول E0
جدیدترین یادداشتها
همه- پایان واکنش
- تولید یک ماده به روشی به جز روش رایج
- فرمول تبدیل واحد های دمایی به هم
- مهندسی برق
- تقطیر جزء به جزء هوا و روش های مختلف صنعتی تهیه گازها
- یه سری درد و دل در باره گاز ها
- شناساگر الکل ها (محلول لوکاس)
- نام و کاربرد برخی از آلیاژ های فولاد
- چند روش کلی برای جداسازی مواد
- کلیپ ازمایش کردن نارنجک دودزای رنگی EG18
بایگانی
- اسفند 1395 2
- آبان 1394 2
- مهر 1394 2
- شهریور 1394 3
- مرداد 1394 20
- تیر 1394 5
- خرداد 1394 3
- اردیبهشت 1394 1
- فروردین 1394 8
- اسفند 1393 6
جستجو
آمار : 176946 بازدید
Powered by Blogsky
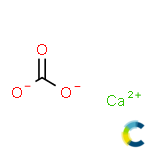
کلسیم کربنات
Substance:Calcium carbonate
(Redirected from Calcium carbonate)
 |  |
- Main ChemSpider page
- Molecular formula: CCaO3
- Molar mass: 100.087
- CAS Registry Number: 1317-65-3
- Appearance: Calcite, naturally occurring mineral, grains, approximately 0.06-0.19in; Calcium carbonate, Puratronic®, 99.997% (metals basis); Aragonite, naturally occurring mineral, grains, approximately 0.06-0.19in; Calcium carbonate, chelometric standard, ACS, 99.95-100.05%; Calcium carbonate, ACS, low in alkalies, 99.0% min; Calcium carbonate, ACS, 99.0% min; Calcium carbonate, 98%; Calcium carbonate, 99.5% (metals basis); Calcium carbonate, 99.95% (metals basis); Calcium carbonate, Puratronic®, 99.99% (metals basis); Calcite, naturally occurring mineral, grains, approximately 0.06-0.19in; Calcium carbonate, Puratronic®, 99.997% (metals basis); Aragonite, naturally occurring mineral, grains, approximately 0.06-0.19in; Calcium carbonate, chelometric standard, ACS, 99.95-100.05%; Calcium carbonate, ACS, low in alkalies, 99.0% min; Calcium carbonate, ACS, 99.0% min; Calcium carbonate, 98%; Calcium carbonate, 99.5% (metals basis); Calcium carbonate, 99.95% (metals basis); Calcium carbonate, Puratronic®, 99.99% (metals basis); Odorless, white powder.; white or colourless crystals or white powder or chunks
- Melting point: 800 to 825 °C
- Boiling point: Not available
- Solubility: 0.001%
- Safety sheet: Not available
- Spectra: Check on SDBS. Add Spectra (Help).
From Wikipedia
Calcium carbonate is a chemical compound with the formula CaCO3. It is formed by three main elements: carbon, oxygen and calcium. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms,snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime, and is created when calcium ions in hard water react with carbonate ions creating limescale. It is commonly used medicinally as a calcium supplement or as an antacid, but excessive consumption can be hazardous.
Read more... Edit at Wikipedia...
Other names
Calcium carbonate (IUPAC Name); Calcium monocarbonate