
علوم کاربردی
آنسوی مرز های شیمی تئوری
علوم کاربردی
آنسوی مرز های شیمی تئوریسرکه یا استیک اسید
Substance:Acetic acid
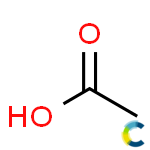 | 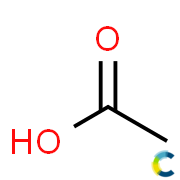 |
- Main ChemSpider page
- Molecular formula: C2H4O2
- Molar mass: 60.052
- CAS Registry Number: 64-19-7
- Appearance: Acetic acid, Acculute Standard Volumetric Solution, Final Concentration 1.0N; Acetic acid, glacial, 99.9+% (metals basis); Acetic acid, Environmental Grade, 99% min; Acetic acid, Environmental Grade Plus, 99.4% min; Acetic acid, glacial, ACS, 99.7+%; Acetic acid, 4% v/v aq. soln.; Acetic acid, 1% v/v aq. soln.; Acetic acid, 0.1N Standardized Solution; Acetic acid, 1.0N Standardized Solution; Acetic acid, glacial, 99+%; Acetic acid, glacial, 99.9985% (metals basis); Acetic acid, Acculute Standard Volumetric Solution, Final Concentration 1.0N; Acetic acid, glacial, 99.9+% (metals basis); Acetic acid, Environmental Grade, 99% min; Acetic acid, Environmental Grade Plus, 99.4% min; Acetic acid, glacial, ACS, 99.7+%; Acetic acid, 4% v/v aq. soln.; Acetic acid, 1% v/v aq. soln.; Acetic acid, 0.1N Standardized Solution; Acetic acid, 1.0N Standardized Solution; Acetic acid, glacial, 99+%; Acetic acid, glacial, 99.9985% (metals basis); Colorless liquid or crystals with a sour, vinegar-like odor. [Note: Pure compound is a solid below 62F. Often used in an aqueous solution.]; clear, colourless liquid/pungent odour
- Melting point: 16.7 °C
- Boiling point: 118 °C
- Solubility: Water, 1e+006 mg/L (25 deg C)
- Safety sheet: Not available
- Spectra: ChemSpider (IR (1, 2, 3), 1H NMR (1, 2), 13C NMR (1, 2), UV), NMRShiftDB 13C NMR, Massbank MS (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24), also check on SDBS. Add Spectra (Help).
From Wikipedia
Acetic acid , systematically named ethanoic acid , is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H or C2H4O2). When undiluted, it is sometimes called glacial acetic acid. Vinegar is roughly 3–9% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell. In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can attack the skin.
Acetic acid is the second simplest carboxylic acid (after formic acid) and consists of two small functional groups, an acetyl group (sometimes symbolized as Ac) and a hydroxyl group (AcOH); it can also be viewed as a methyl group and a carboxyl group linked. It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. As a food additive it is approved for usage in many countries, including Canada, the European Union, the United States, Australia and New Zealand. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats.
The global demand for acetic acid is about 6.5 million metric tons per year (Mt/a), of which approximately 1.5 Mt/a is met by recycling; the remainder is manufactured from petrochemical feedstock. As a chemical reagent, biological sources of acetic acid are of interest, but generally cannot compete economically. Vinegar is mostly dilute acetic acid, often produced by fermentation and subsequent oxidation of ethanol.
Read more... Edit at Wikipedia...
Acetic acid, also known as vinegar, is used as a medication to treat a number of conditions. As an eardrop it is used to treat infections of the ear canal. It may be used with an ear wick. As a gel it may be used to adjust the pH of the vagina. It may also be applied to the cervix to help detect cervical cancer during screening.
Side effects may include burning at the site of application. Allergic reactions may rarely occur. Use is not recommended in the ear in people who have a hole in the eardrum. It works against both bacterial and fungal causes of external ear infections.
Acetic acid has been used medically since the time of Ancient Egypt. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Acetic acid is avaliable as a generic medication. In the United States a course of treatment with the ear preparation costs less than 25 USD. Acetic acid is more commonly used for external ear infections in the developing world than the developed.
Read more... Edit at Wikipedia...
Other names
Acetic acid (IUPAC Name); Ethanoic acid
References
Please add any relevant references here.